On Wednesday, Johnson & Johnson announced the results of a clinical trial showing that its experimental vaccine can match the antibodies produced by other vaccines in two doses with only one dose, and can induce an immune response for at least 71 days after vaccination; when the volunteers were vaccinated for the second time 2 months later, the number of immune system factors, called neutralizing antibodies, had increased significantly – by three times!
The results prove the power of medical innovation – if only one dose is enough to produce sufficient antibodies to the coronavirus, this will greatly simplify the task of vaccination and hopefully achieve herd immunity in a shorter time. In addition, the storage conditions of the vaccine developed by Johnson & Johnson are not as strict as other vaccines (such as Moderna and Pfizer), and therefore according to the company storage and logistics will not face as many problems as other vaccines. For example, the J&J vaccine can be stored in the refrigerator for up to three months; the Moderna and Pfizer/BioNTech vaccines need to be frozen and stored at minus 20 degrees and minus 70 degrees Celsius respectively. Additionally the Moderna and Pfizer/BioNTech vaccines require two shots, which means repeat shipping and clinic visits.
According to Stoffels, chief scientist at Johnson & Johnson, the final stage efficacy data for 45,000 volunteers is expected to be available in early February. If the evaluation result shows that a single vaccination can continue to produce antibodies in the body, with an effectiveness of 60% or more (generally, any coronavirus vaccine with an effectiveness of more than 50% is considered a success), and can even effectively defeat new mutant strains, Johnson & Johnson is expected to receive emergency use authorization from the end of February to March.
It is worth noting that the New York Times quoted people familiar with the matter as saying that Johnson & Johnson had already experienced serious delays in vaccine production and fallen behind the original schedule by as much as two months. It is reported that the company should have delivered more than 60 million doses of vaccine by the end of April. In the face of huge market demand, Johnson & Johnson, with limited resources, may not be able to supply the government with the promised number of doses in the scheduled time.
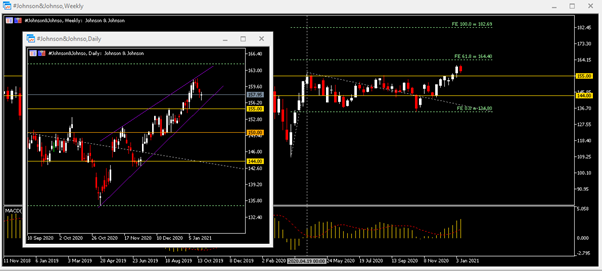
From a technical analysis point of view, #Johnson&Johnson successfully broke through last April’s high ($157) last week, and the MACD lines remained on the neutral zone. The daily chart shows that the stock price briefly refreshed above US $161.30 this Monday (January 11) and then fell under pressure, and is still trading in a rising wedge. The trend line lies close to the $155 near-term support. If there is a decisive break of this support level, the focus turns to $150 and $144 support level. On the contrary, the recent high ($161.30) and the wedge-shaped trend line are the key resistances. The second resistance is the 61.8 Fibonacci extension level ($164.40) and the third resistance is the 100.0 Fibonacci extension level ($182.70).
The final stage efficacy data in early February will greatly affect the future trend of the stock price. In addition, whether the company can deliver the required vaccines within the scheduled time in the follow-up process is also a concern for investors. Any related issues such as delays in vaccination, reduced effectiveness, insufficient storage, logistics, and human resources will affect the results, and in turn the infection rates, mortality, and subsequent economic recovery, increasing the time required to achieve a full recovery to pre-crisis levels.
Click here to access the HotForex Economic Calendar
Larince Zhang
Market Analyst
Disclaimer: This material is provided as a general marketing communication for information purposes only and does not constitute an independent investment research. Nothing in this communication contains, or should be considered as containing, an investment advice or an investment recommendation or a solicitation for the purpose of buying or selling of any financial instrument. All information provided is gathered from reputable sources and any information containing an indication of past performance is not a guarantee or reliable indicator of future performance. Users acknowledge that any investment in Leveraged Products is characterized by a certain degree of uncertainty and that any investment of this nature involves a high level of risk for which the users are solely responsible and liable. We assume no liability for any loss arising from any investment made based on the information provided in this communication. This communication must not be reproduced or further distributed without our prior written permission.